Abstract
Introduction
Autologous adoptive T cell therapies, based on the use of tumor infiltrating lymphocytes (TILs), have made great progress in recent years for the treatment of solid tumors, especially melanoma. However, further work is needed to isolate tumor-reactive T cells among patients diagnosed with hematologic malignancies. The dynamics of the interaction between T cells and antigen presenting cells (APC) dictate the quality of the immune responses. While stable joints between target cells and T lymphocytes lead to the induction of T cell activation and immune response, brief contacts contribute to the induction of immune-tolerance. Taking advantage of the strong interaction between target cell and activated T-cells, we show the feasibility to identify and isolate tumor-specific cytotoxic T lymphocytes (CTLs) from acute myeloid leukemia (AML) patients. Using this approach, CTLs stably bound through T cell receptor to tumor cells (doublet forming T-cells) can be identified in peripheral blood and bone marrow and subsequently selected and isolated by FACS-based cell sorting.
Methods
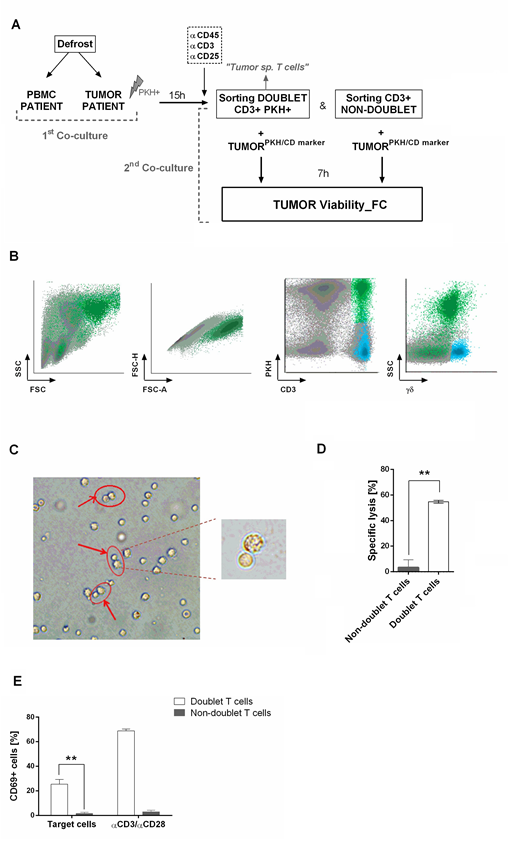
Co-cultures between PBMC from AML patients in complete remission and AML tumor cells (PKH-stained) from the same patient were performed to study the percentage of doublet-forming T cells (CD3+PKH+) (T cell bound to a tumor cell). After 15 hours of co-culture, cells were stained and sorted. Secondary co-cultures with autologous tumor cells (used in primary co-culture) were performed to study the cytotoxic activity and cytokine production of T-cells capable or not to form stable joints with the leukemic cells (doublet population vs non-doublet population).
Results
Doublet-forming T cells from AML patients were identified in a range of 2% to 6% (mean=3.83%, n=5). Immunophenotyping analysis showed differences between doublet-forming T cells (CD3+PKH+) and those T cells which did not form stable and strong interactions with target cells (CD3+PKH-). Doublet T cells displayed a higher percentage of CD8+ T cells and higher percentage of effector CD4+ and CD8+ T cells compared to non-doublet T cells. Next, we explored, among effector CD4+ and CD8+ cells, those with cytotoxic phenotype. As expected, a high percentage of effector CD8+ doublet T cells showed Granzyme B and perforin expression, thus corresponding with a cytotoxic immune-phenotype (n=3, mean 65.51%). Within effector CD4+ doublet T cells, a mean of 9.053 % showed expression of both Granzyme B and perforin corresponding with CD4+ CTL (n=3). Regarding CD57 and CD16 markers, a mean of 18.62% of effector CD4+ doublet T cells were positive for both markers, compared to 65.84% of effector CD8+ doublet T cells (n=3).
Further, we performed secondary co-cultures to analyze the CD69 activation marker after 24h of co-culture. A high percentage of CD69+ cells was observed in co-cultures with doublet-forming T cells against target cells as compared to non-doublet T cells (n=3, p=0.0053). Finally, analysis of supernatants of co-culture of doublet T cells and non-doublet T cells with target cells revealed specific secretion of IFNγ and IL-2 (n=3, p=0.0001; p=0.0005, respectively).
The cytolytic activity was evaluated comparing the viability of tumor cells cultured alone or with doublet-forming T cells or non-doublet T cells from the same patient. A significant increase of the specific lysis of AML cells was observed when doublet T cells were co-cultured as compared to non-doublet T cells (p=0.0424, n=5). This encouraged us to examine whether we were able to identify doublet-forming T cells from bone marrow of AML patients at diagnosis. Analyses of bone marrow by flow cytometry reveled a small percentage of CD3+CD34+ population corresponding with bone marrow-doublet-forming T cells (n=3, mean=2.9%). Interestingly, bone marrow-doublet-forming T cells show a higher percentage of CD4+ T cells, whereas bone marrow-non-doublet T cells show a higher percentage of CD8+ T cells.
Conclusions
Our data demonstrate that when T cells from AML patients are co-cultured with tumor cells, a "doublet T cell" population appears. This population consists of T cells capable to bind tumor cells. These CTLs display higher percentage of effector cells and a marked cytotoxic activity against AML blasts. In conclusion, we have developed a new procedure to identify and select specific cytotoxic T cells in both bone marrow and peripheral blood from patients diagnosed with acute myeloid leukemia.
Sanchez-Abarca:Virgen del Rocio University Hospital: Patents & Royalties. Ramos:Takeda Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal